Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste Small amounts of gaseous hydrogen peroxide occur naturally in the air Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat Although nonflammable, it is a powerful oxidizing agent that can cause spontaneous combustion when it Hydrogen is the simplest and most abundant element on earth—it consists of only one proton and one electron Hydrogen can store and deliver usable energy, but it doesn't typically exist by itself in nature and must be produced from compounds that contain itHydrogen is a chemical element – one of the building blocks that all matter in the universe is made of Of all the elements, it is the simplest and most abundant Down on Earth, finding hydrogen gas in nature is very rare However, hydrogen is reactive and

Bronkhorst Its Share Of A Clean Hydrogen Energy Future Bronkhorst
Hydrogen in nature
Hydrogen in nature- Hydrogen Bond Definition A hydrogen bond is a type of attractive (dipoledipole) interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom This bond always involves a hydrogen atom Hydrogen bonds can occur between molecules or within parts of a single molecule A hydrogen bond tends to be stronger How this works is that the polar nature of the water molecule means each hydrogen atom experiences attraction to both the oxygen it's bound to and to the nonhydrogen side of the oxygen atoms of other water molecules Hydrogen bonding in water results in the crystal structure of ice, making it less dense than water and able to float




Hydrogen Isotopes Springerlink
There is more hydrogen in the universe than any other element—it's been estimated that approximately 90 percent of all atoms are hydrogen But hydrogen atoms do not exist in nature by themselves To produce hydrogen, its atoms need to be decoupled from other elements with which they occur— in water, plants or fossil fuels Arising from the nature of the hydrogen bond and otherfactors, such as the disordered arrangement of hydrogenin water, the unusual properties of H2O have madeconditions favorable for life on Earth The way in which a compound inspired by nature produces hydrogen has now been described in detail for the first time by an international research team from the University of Jena, Germany and the
Protium (1 H), deuterium (2 H) and tritium (3 H) These isotopes form naturally in nature Protium and deuterium are stable Tritium is radioactive and has a halflife of about 12 years Scientists have created four other hydrogen isotopes (4 H to 7 H), but these isotopes are very unstable and do not exist naturallyThe main isotopes of hydrogen are uniqueHydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form, it is a very pale blue liquid, slightly more viscous than waterIt is used as an oxidizer, bleaching agent, and antisepticConcentrated hydrogen peroxide, or "hightest peroxide", is a reactive oxygen species and has been used as a propellant in rocketryIts chemistry is dominated by the O–O bond Not present in nature alone, hydrogen can be produced through a process of electrolysis, the splitting of the molecules in water, with electricity from renewable energy sources like wind and solar This type of hydrogen is called 'green', as the process does not emit any greenhouse gas emissions to the environment
The nature of hydrogen in x‐ray photoelectron spectroscopy General patterns from hydroxides to hydrogen bonding After a select review of the infrequent use made by others to attribute XPS peak shifts to hydrogen bonding, we consider in some detail two cases recently published by members of our groupHowever, it became too dangerous because of its highly flammable nature Hydrogen gas can be produced in a lab by combining a dilute acid with a metal More on the Elements and the Periodic Table Elements Periodic Table Alkali Metals Lithium Sodium Potassium Alkaline Earth Metals Beryllium Magnesium Calcium Radium Transition MetalsHydrogen sulfide is commonly found in raw natural gas and biogas It is typically removed by amine gas treating technologies In such processes, the hydrogen sulfide is first converted to an ammonium salt, whereas the natural gas is unaffected RNH 2 H 2 S ⇌ RNH




Can The Oil And Gas Industry Deliver Canada S Hydrogen Strategy




Hydrogen S Potential Is Not Just Hot Air Wsp
Hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron The hydrogen nucleus is made up of a particle carrying a unit positive electric charge, called a proton The isolated hydrogen ion, represented by the symbol H , is therefore customarily used to represent a protonHydrogen bonding is an important factor in determining the 3D structures and properties that are acquired by synthetic and natural proteins Hydrogen bonds also play an important role in defining the structure of cellulose as well as derived polymers such as cotton or flax Strength of the Hydrogen bond The hydrogen bond is a weak bond The vast majority of hydrogen in use — and there is plenty of it, mainly in industry — is made from natural gas The process emits CO 2 Second, 'blue', or




Hydrogen Element Information Properties And Uses Periodic Table




Influence Of The Nature Of Ib Group Metals On Catalytic Activity In Reactions Of Homomolecular Hydrogen Exchange On Cu Ag Au Nanoparticles Sciencedirect
One of the easiest ways to obtain hydrogen is to get it from water, H 2 O This method employs electrolysis, which breaks water into hydrogen and oxygen gas Hydrogen has three isotopes These are protium ,deuterium and tritium The most abundant isotope of hydrogen is protium % is deuteriumIt is mostly in the form of HD Tritium being unstable because of its radioactive nature occurs only in traces Solid hydrogen, a simple system consisting only of protons and electrons, exhibits a variety of structural phase transitions at high pressures




Comprehensive Review On Natural Hydrogen Shows That It Has Been Underestimated World Energy




Hydrogen Wikipedia
This documentary tells the story of hydrogen through a series of intriguing interviews from those working on cuttingedge hydrogen technologies The programHydrogen is an essential for life, the universe and just about everything Life, in fact, is multiply dependent on it Without hydrogen we wouldn't have the Sun to give us heat and light There would be no useful organic compounds to form the building blocks of life And that most essential substance for life's existence, water, would not exist Hydrogen Water Side Effects – My Conclusion The daily consumption of water rich in hydrogen is a potentially new therapeutic strategy to improve the quality of life The consumption of water rich in hydrogen can reduce the biological response to oxidative stress




Nature Com Reports Hydrogen Sulfide Conducts Electricity With Zero Resistance Analytical Technology Inc 1 800 959 0299




Hydrogen Isotopes Springerlink
Disperses quickly Is nontoxic Produces water upon combustion Can be stored safely Does not plume Does not leach Is stable in ambient temperaturesHydrogen has three main isotopes;Only about 1% by mass of hydrogen is required in the Earth's core to explain the density deficit, and this corresponds to a hydrogen–iron mass ratio that is only about onetenth of that present in




The New Alchemy Turning Plastic Into Hydrogen News Nature Middle East




Yara Ready To Enable The Hydrogen Economy With Historic Full Scale Green Ammonia Project World Business Council For Sustainable Development Wbcsd
The presence of hydrogen at high concentrations up to 58% by volume in free gas phase, has been found in extensive areas in some gold mines in South Africa, Canada and Finland a several hundreds Hydrogen has about a third of the energy content of natural gas, requiring modifications all along the gas system If it is green hydrogen, as opposed to a blend, these modifications are going to When hydrogen (H), the lightest, smallest and most abundant atom in the Universe, makes its way into a highstrength alloy (strength above ~650 MPa), the




Renewable Hydrogen Key To A New Civilization Our World



Hydrogen Sulphide How Nature Uses It And Dredges Get Rid Of It Discover Dredging
Credit Nature Communications /s A new method of extracting hydrogen from water more efficiently could help underpin the capture of renewable energy in the form of Hydrogen — with just one proton and one electron (it's the only element without a neutron) — is the simplest element in the universe, which explains why it'sHydrogen Hydrogen is a widely available gas through delivery from a refinery or can be produced onsite by way of utilizing other renewable energy sources to convert water or other natural gases into hydrogen From Encyclopedia of Electrochemical Power Sources, 09 Download as PDF




Hydrogen Sulfide H2s Picarro




Producing Hydrogen Using Less Energy Eurekalert Science News
But one of the final hurdles to hydrogen power is securing a safe method for detecting hydrogen leaks A new study published in Nature Communications documents an inexpensive, sparkfree, optical Hydrogenbased fuels should primarily be used in sectors such as aviation or industrial processes that cannot be electrified, finds a team of researchers Nature Climate Change, DOIWater (H2 O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, nearly colorless with a hint of blueThis simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the "universal solvent" for its ability to dissolve many substances This allows it to be the "solvent of life" indeed, water as found in nature




Green Recovery Mps Call For Vat Breaks For Energy Efficiency Electric Vehicles And Circular Economy
:strip_icc()/i.s3.glbimg.com/v1/AUTH_59edd422c0c84a879bd37670ae4f538a/internal_photos/bs/2021/a/p/THh95zTtioq01k3AeTYA/imagem1-1-.jpg)



Green Hydrogen The 6 Countries Leading The Production Of The Fuel Of The Future Nature
Hydrogen occurs naturally on earth only in compound form with other elements in liquids, gases, or solids Hydrogen combined with oxygen is water (H2O) Hydrogen combined with carbon forms different compounds—or hydrocarbons—found in natural gas, coal, and petroleum Main Hydrogen is one of the basic materials in science, and many major discoveries have been made from studies of atomic and molecular hydrogen Dense hydrogen has been ofHydrogen (1 H) has three naturally occurring isotopes, sometimes denoted 1 H, 2 H, and 3 H The first two of these are stable, while 3 H has a halflife of 1232 years There are also heavier isotopes, which are all synthetic and have a halflife less than one zeptosecond (10 −21 second) Of these, 5 H is the most stable, and 7 H is the least




The Peculiar Nature Of Molecular Hydrogen Vibration Under High Pressure




Is Hydrogen Rich Water Good For You Water Hydrogen Water Water Benefits
Answer to If the average atomic mass of hydrogen in nature is amu, what does that tell you about the percent composition of H1 and H2 in Hydrogen bonds are weak, generally intermolecular bonds, which hold much of soft matter together as well as the condensed phases of water, network liquids, and many ferroelectric crystals The small mass of hydrogen means that they are inherently quantum mechanical in nature, and effects such as zeropoint motion and tunneling must be considered, though all too Pure hydrogen gas rarely occurs in nature, although volcanoes and some oil wells release small amounts of hydrogen gas Hydrogen is in nearly every compound in the human body For example, it is in keratin, the main protein that forms our hair and skin, and in the enzymes that digest food in our intestines




Renewable Hydrogen Through Concrete And Clay Nature Will Find A Way




Gruber Logistics Pioneering The Introduction Of Hydrogen Trucks On European Roads Fuelcellsworks
The fuel cell process of converting hydrogen back to electricity is only 60% efficient, after which you have the same 5% loss from driving theAbout percent of the hydrogen in nature is protium The man who gave hydrogen its name, AntoineLaurent Lavoisier, is sometimes called the father of modern chemistry Hydrogen2 is known as deuterium A deuterium atom contains one proton, one electron, and one neutron




How Green Hydrogen Can Become A Cost Competitive Climate Solution Earth Org Past Present Future




A Seamless Hydrogen Supply Chain



Researchers Discover Natural Way To Produce Hydrogen Fuel




Thank A House Plant Hydrogen Based Energy Storage Inspired By Mother Nature Cleantechnica




Hydrogen Production And Applications Of Hydrogen Britannica




Hydrogen Peroxide Hero Or Villain Faithful To Nature




The Nature Of The Hydrogen Bonded Chains In The High Pressure P2 1 C Download Scientific Diagram




National Park Waterfall Nature Play Iceland Gusher Hydrogen Sulphide Scenery Stock Photo Alamy




Hydrogen Squeezed From Stone Could Be New Energy Source c News



1



Anu Unveils Solar To Hydrogen Cells Made Of Silicon And Perovskite With Conversion Efficiency Of 17 6 The Leading Solar Magazine In India




Experience From Previous Transitions Are Useful For Hydrogen Gasterra




Gazprom Proposed To Build A Hydrogen Production Plant In Germany Eurasia Network




New Type Of Ultra Strong Chemical Bond Discovered Live Science




Land Rover S Defender Will Use Hydrogen Fuel Cells Nature News Express Co Uk




Canada Unveils Hydrogen Strategy To Kick Start Clean Fuel Industry Mining Com




Bronkhorst Its Share Of A Clean Hydrogen Energy Future Bronkhorst




Why Is Hydrogen Written H2 Instead Of H Quora




Hydrogen Key Technology Of The Future Youtube




Hydrogen H2 Letters On A Meadow With Flowers And One Orange Butterfly In The Nature Stock Photo Alamy




1 Dipolar Nature Of Water Molecule And Intermolecular Hydrogen Bond Download Scientific Diagram




Hydrogen The Future Of Zero Carbon Fuels




Catalyst Fuels Hydrogen Car Vision Research Chemistry World



1




Ctc Bonn Only With Hydrogen Can We Save The Climate Fuelcellsworks




Hydrogen Oxygen High Res Stock Images Shutterstock



Hopes After New Hydrogen Production Method Revolution Green



New Hydrogen Sensor Takes Its Inspiration From Nature
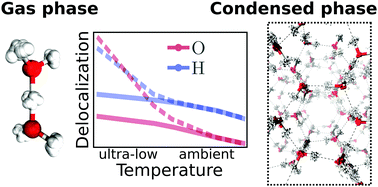



Quantum Nature Of The Hydrogen Bond From Ambient Conditions Down To Ultra Low Temperatures Physical Chemistry Chemical Physics Rsc Publishing



Scientists Discover New Soar Energy Hydrogen Production Method




Baker Hughes And Novatek To Develop Low Carbon Hydrogen Solution For Lng Trains




Repurposing Gas Infrastructure For Hydrogen Siemens Energy Global
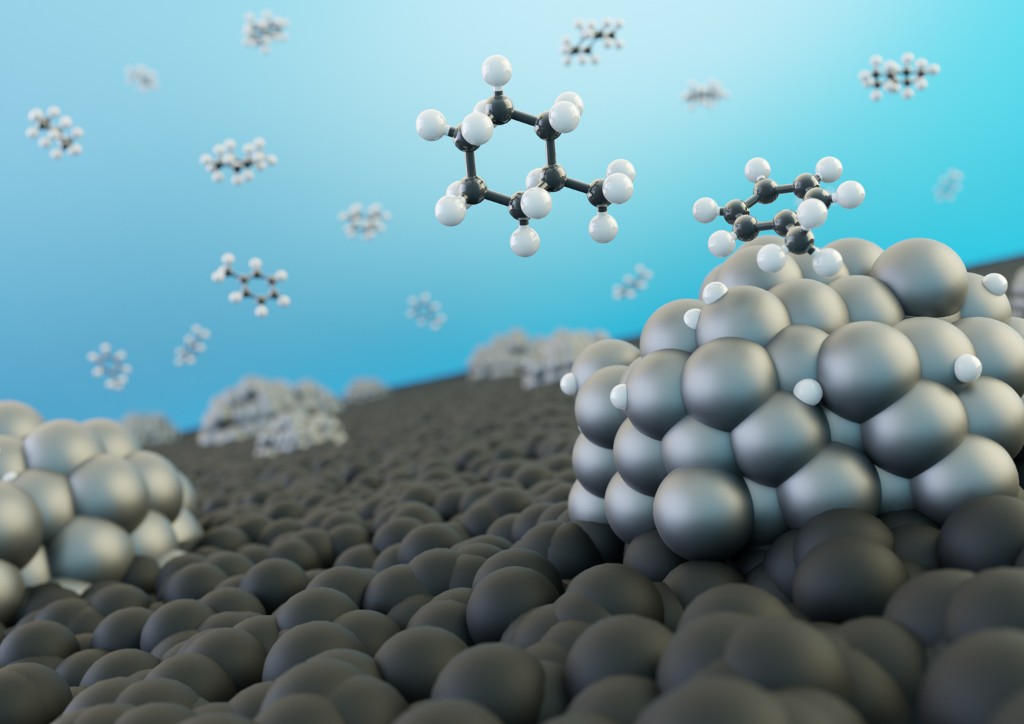



A Hydrogen Powered Society Is Around The Corner




Hydrogen Why Do We Need It Cph2




Kering S Fund For Nature And A New Green Hydrogen Plant The Sustainability Success Stories Of The Week




The Nature Of The Hydrogen Bond Outline Of A Comprehensive Hydrogen Bond Theory International Union Of Crystallography Monographs On Crystallography Book 23 Illustrated Gilli Gastone Gilli Paola Amazon Com




Pipeline Owners Look To Hydrogen As Natural Gas Comes Under Attack




Electrostatics Charge Transfer And The Nature Of The Halide Water Hydrogen Bond Physical Chemistry Chemrxiv Cambridge Open Engage




48 Zenii Hydrogen Solutions Ideas In 21 Molecular Solutions Hydrogen Water




The Essential Element Surrounded By Hydrogen Joi Scientific
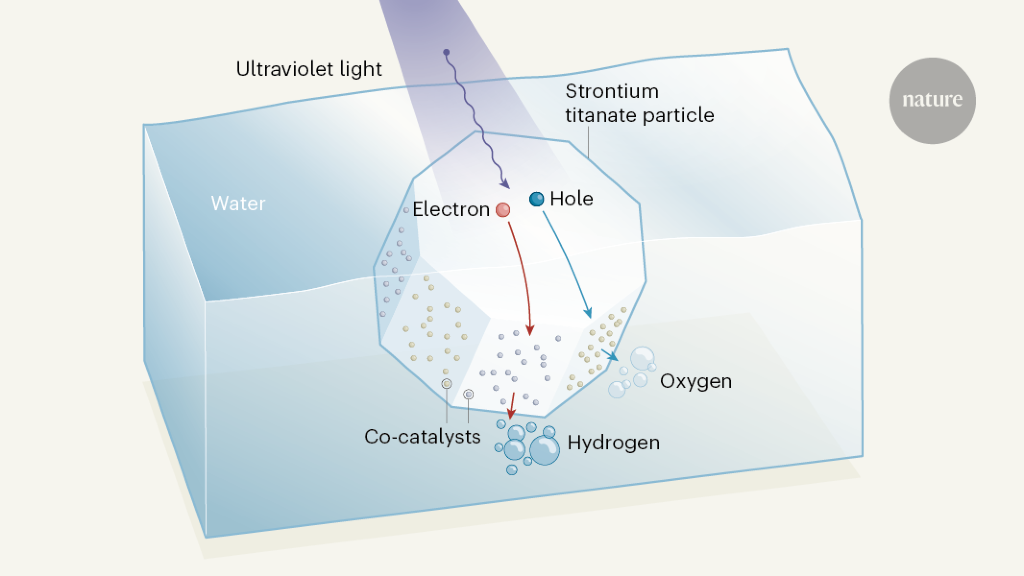



An Almost Perfectly Efficient Light Activated Catalyst For Producing Hydrogen From Water
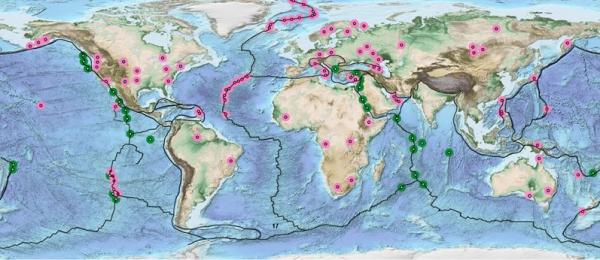



Natural Hydrogen A Geological Curiosity Or The Primary Energy Source For A Low Carbon Future Renewable Matter




Pgm Based Fuel Cells Applications For Industry New Age Metals Inc




How Hydrogen Can Spark The Next Zero Emissions Revolution Ey Us




Hydrogen As A Fuel Learning From Nature 1st Edition Richard Camm




Incentives For Hydrogen Powered Or Electric Cars In Energyrates Ca



Hydrogen Chemical Element Reaction Water Uses Elements Examples Metal Gas Number




587 Element Hydrogen Photos Free Royalty Free Stock Photos From Dreamstime




Hydrogen Nature S Fuel Introduction And Fuel Cells Pbs Learningmedia
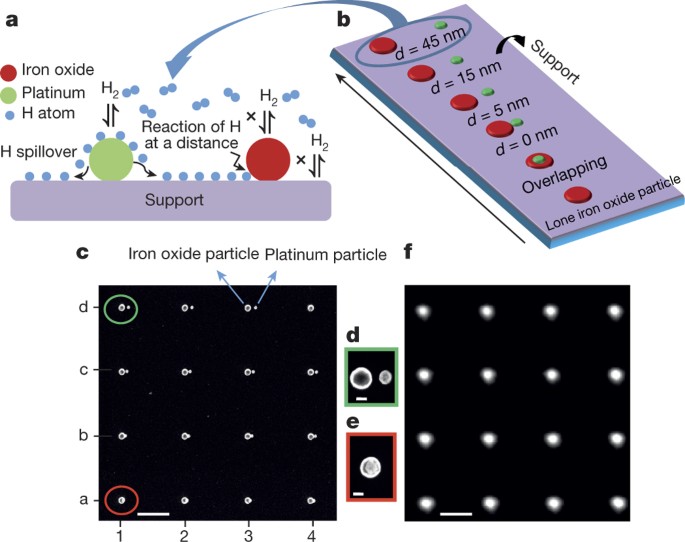



Catalyst Support Effects On Hydrogen Spillover Nature




China Zeolite Molecular Sieve 5a For High Purity Nitrogen Oxygen Hydrogen And Nature Gas Inert Gases Produce China Molecular Sieve Molecular Sieve 5a




Carbon Zeolite Molecular Sieve 4a For Chemical Production Of High Purity Nitrogen Oxygen Hydrogen And Nature Gas Inert Gases Buy 4a Zeolite 4a Zeolite For Chemical Carbon Molecular Sieve 4a Product On Alibaba Com




Vaillant Uk Are You Ready For A Future Heated By Hydrogen At Vaillant We Pride Ourselves On Being At The Forefront Of Technology Which Is Why We Are Already Developing Hydrogen
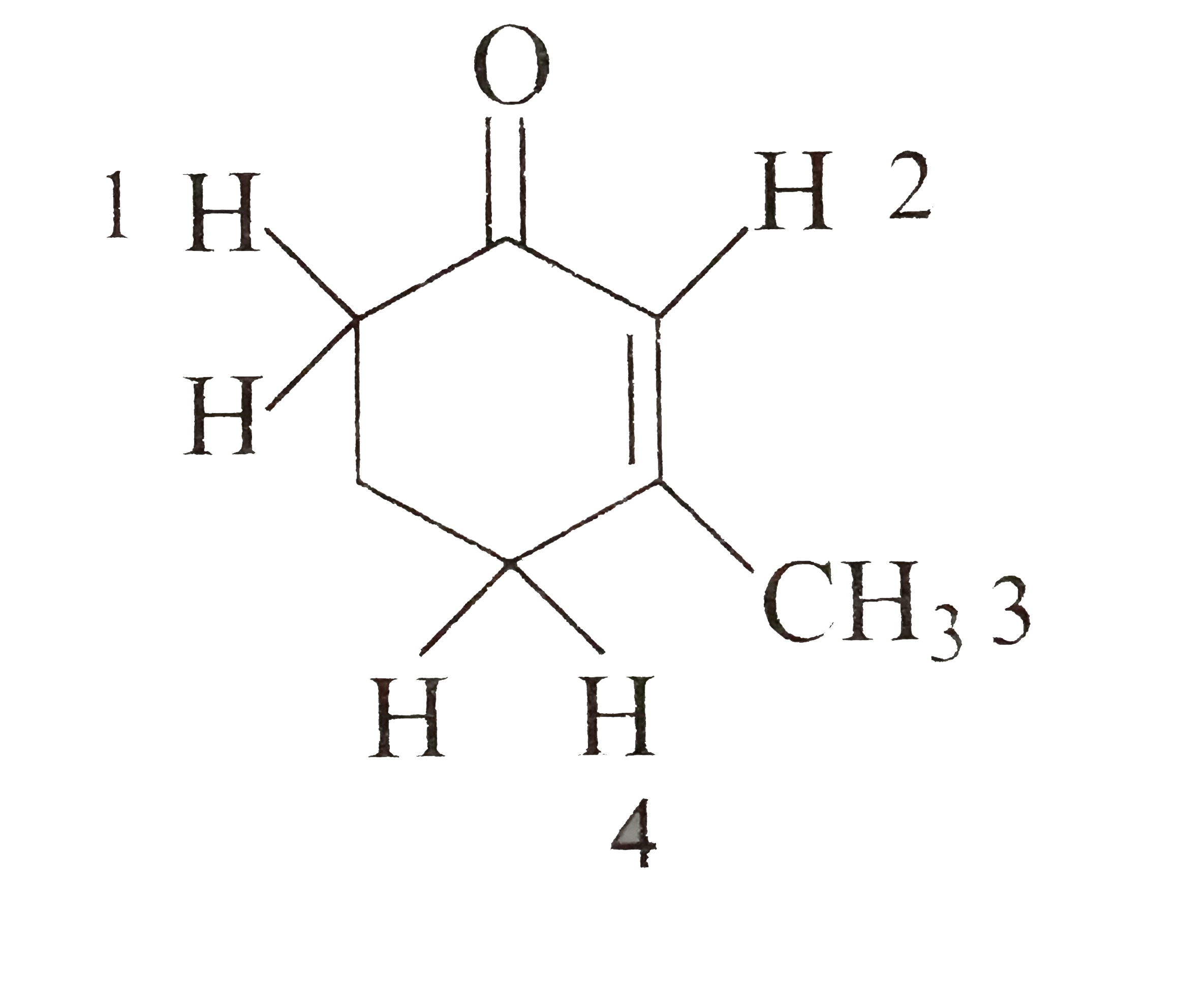



Which Hydrogen Of The Given Compound Is Least Acidic In Nature




Hydrogen Basics Ways2h




Material Makes Hydrogen Gas 10 Times Faster Than Natural Enzyme Uses Inexpensive Metals Nextbigfuture Com




15 Things You Need To Know About Hydrogen Tno




The Boat Circling The Planet On Renewable Energy And Hydrogen Wired




Front Cover The Nature Of Hydrogen Bonds A Delineation Of The Role Of Different Energy Components On Hydrogen Bond Strengths And Lengths Chem Asian J 16 19 Van Der Lubbe 19




First View Of Nature Inspired Catalyst After Ripping Hydrogen Apart Provides Insights For Better Cheaper Fuel Cells




Bullet Proof Armour And Hydrogen Sieve Add To Graphene S Promise Nature News Comment



Hydrogen Sulfide Creationwiki The Encyclopedia Of Creation Science




Molecular Hydrogen Oxidative Stress Hydrogen Technologies




Scientists Find Cheaper Way To Make Hydrogen Energy Out Of Water
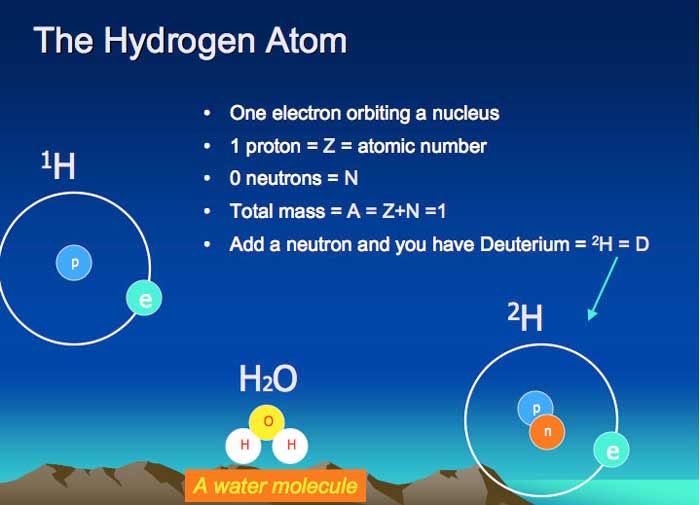



Hydrogen Preparation Isotops Hydrogen Uses And Properties




The Forever Energy The Unmatched Clean Energy Potential Of Hydrogen Joi Scientific




Pdf Natural Hydrogen Exploration Guide



1
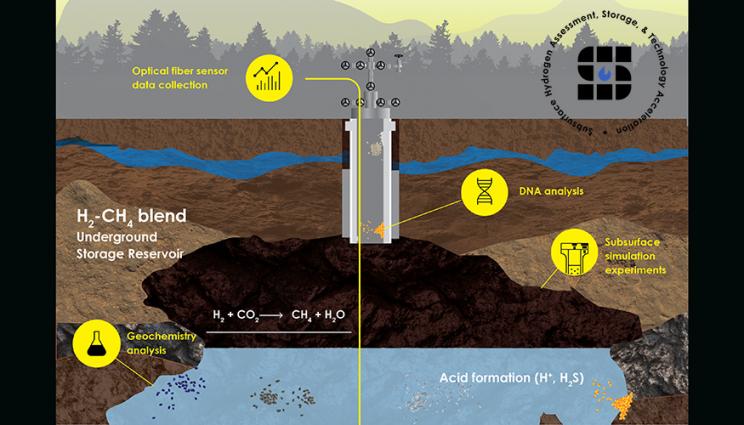



Gxwdponkhlmuwm



Article The Missing Fuel For Our Body Avocadoninja




Hydrogen H2 Letters On A Meadow With Flowers And One Orange Butterfly In The Nature Stock Photo Alamy




5 E Hydrogen Bonding Nature Of The A B Sheet And B A Helix Hydrogen Download Scientific Diagram




Hydrogen Cloud Natural Diamonds Nature Clouds




The Truth About Hydrogen Rmi




Hydrogen Fuel From Sunlight Berkeley Lab




Anu Researchers Nature Offers Potential For Endless Renewable Hydrogen Fuelcellsworks
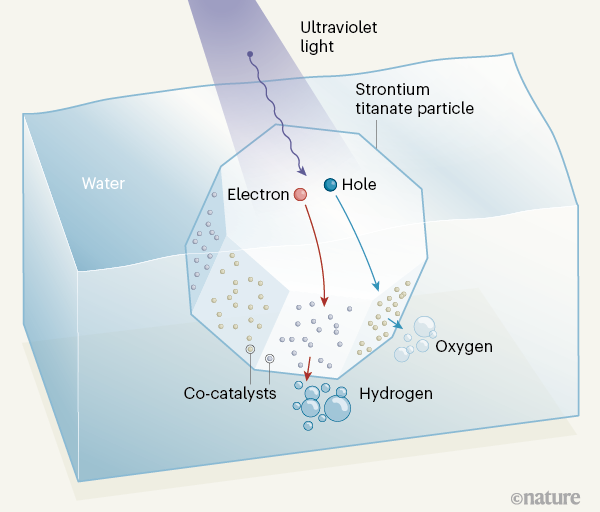



An Almost Perfectly Efficient Light Activated Catalyst For Producing Hydrogen From Water




Electric Drive Car Stickfigure Nature Hydrogen Iphone Case Flexible Spreadshirt
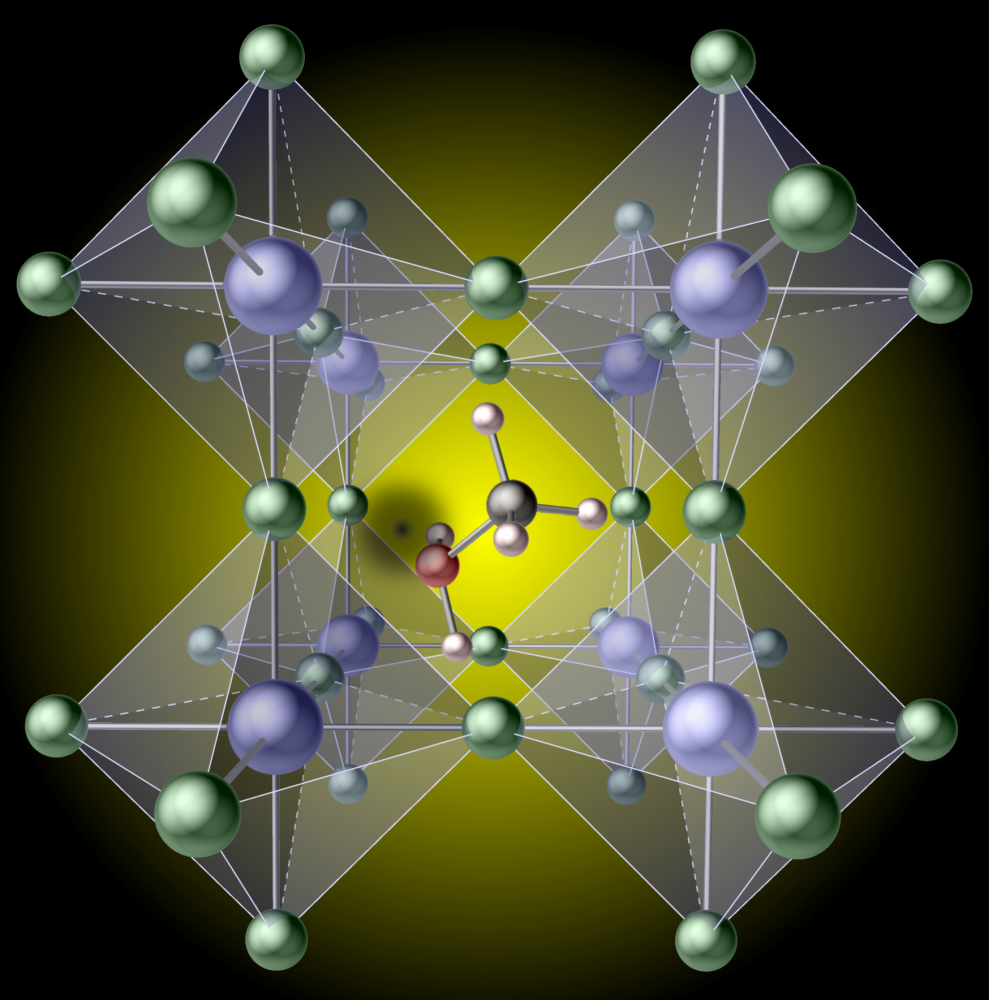



Less Innocent Than It Looks The Ucsb Current




Hydrogen Nature World News




Saving Nature Scotland Will Soon Have Its First Hydrogen Powered Train Scotland Times Of India Travel



1




Everything You Need To Know About Green Hydrogen And Why It Attracts Investors
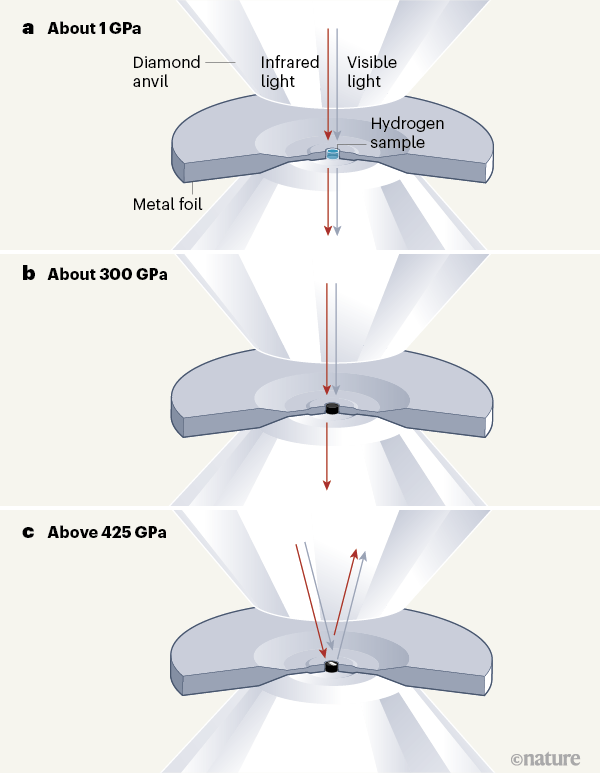



A Milestone In The Hunt For Metallic Hydrogen



0 件のコメント:
コメントを投稿